Hemp oil extracts containing CBD oil have just been banned by the FDA. Before I can explain just how horrendous this is, and the insane way their justifying it, let me explain what CBD oil is.
CBD is one of over 60 compounds found in cannabis that belong to a class of molecules called cannabinoids. Of these compounds, CBD and THC are usually present in the highest concentrations, and are therefore the most recognized and studied.
CBD is non-psychoactive because it does not act on the same pathways as THC.These pathways, called CB1 receptors, are highly concentrated in the brain and are responsible for the mind-altering effects of THC.A 2011 review published in Current Drug Safety concludes that CBD “does not interfere with several psychomotor and psychological functions.” The authors adds that several studies suggest that CBD is “well tolerated and safe” even at high doses.
The Medical Benefits
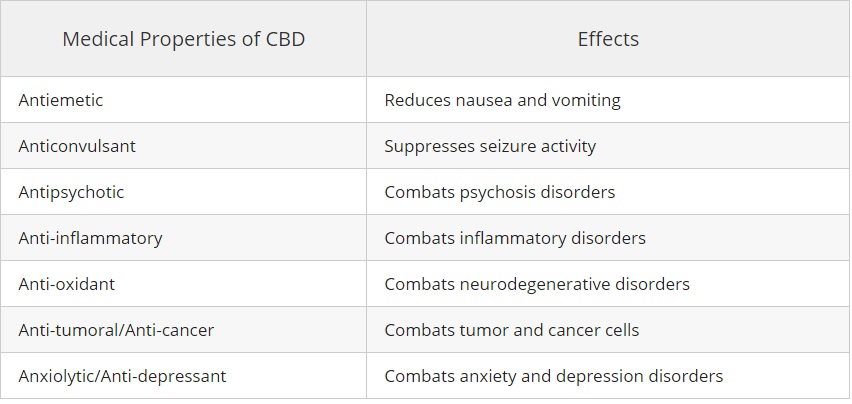
According to a 2013 review published in the British Journal of Clinical Pharmacology, studies have found CBD to possess the following medical properties:
The CBD oil that you were able to purchase online and around the US, before the ban, was derived from hemp oil and not marijuana. Because Hemp is not technically federally illegal – those there are massive regulations on growing it – the extract from it were considered legal.
Hemp is used for making medicinal remedies, food, fiber, rope, paper, bricks, oil, natural plastic, and so much more. Whereas marijuana is usually used just recreationally, spiritually, and medicinally.
FDA has now outlawed the sale of Hemp derived CBD Oil
Let’s break it down numerically
1.) It has been demonstrated that people with anxiety, seizures, cancer, psychosis, neuro-degenerative disorders, AIDS, and depression are helped with with CBD oil.
2.) Pharmaceutical companies want to “prove this” to the FDA in order to market it and sell it.
3. ) As a result, the FDA is now banning the sale of it, leaving the drug into testing limbo for years, so that the pharmaceutical companies can then repackage it and sell it to you for outrageous price.
4. ) All the while, millions of people who could be helped with CBD oil will be made criminals if they try to get their hands on this natural product.
The FDA can do this to any vitamin or supplement
They are also claiming that because these companies state that CBD has a medicinal benefit – which it has been shown to have – these companies are breaking the law.
What must be understood is that this power that the FDA has, to monopolize natural products, ensures that at any point they can take a product off the shelves. A pharmaceutical company can then patent it, and make millions of something that you can make at home.
Possibly never. Let me explain:
In the United States, it takes an average of 12 years for an experimental drug to travel from the laboratory to your medicine cabinet. That is, if it makes it. Only 5 in 5,000 drugs that enter preclinical testing progress to human testing. One of these 5 drugs that are tested in people is approved.
Why the FDA needs to be abolished
What I’ll leave you with is a biomedical researcher whistle-blower’s explanation of why we need to get rid of the FDA:
As many as 1 out of 3 people who have died from disease in the last 40 years did so needlessly because of a single law passed by Congress in 1962! Here’s my “insider” story.
For 19 years, I was a research scientist with the Upjohn Company, a mid-sized pharmaceutical company. I once joked that we were so busy complying with superfluous regulations, we had little time to discover new drugs. Unfortunately, it’s no laughing matter.
In 2003, enough studies had been published on the 1962 Kefauver-Harris Amendments to estimate the true cost of these FDA regulations. Researchers had long suspected that they had thwarted innovation, driven up drug prices, and delayed the introduction of life-saving pharmaceuticals.
Prior to the passage of these Amendments, the FDA primarily regulated only drug safety. The Amendments gave the FDA authority over drug manufacturing, advertising, animal studies, and the design of clinical trials.
The result was predictable: the time it took to take a drug from the laboratory to the market went from 4 years to 14 years. Because patent life was 19 years or less, manufacturers had insufficient time to recover their costs before a drug went generic.
In 1984, Congress passed the Waxman-Hatch Act, which partially restored the patent years destroyed by regulation. The act estimated that regulations were responsible for a whopping 84% of the 14-year development time. Prior to 1962, about 15% of the development time was consumed by regulatory requirements.
When the AIDS epidemic arose, pharmaceutical companies began to develop treatments. However, most AIDS patients couldn’t wait the 14 years that it then took to get through the regulatory red tape. A small group of concerned activists hired underground chemists to make the very drugs that we were working on.
By the time the FDA gave us permission to test our new drugs in people, virtually the entire AIDS community had already received them. Since the regulatory testing had to be done in people who hadn’t yet received the drug, we had to wait for new cases to be diagnosed.
Although the actions of the AIDS activists were illegal, neither the FDA nor the pharmaceutical companies chose to prosecute. Indeed, the AIDS community demonstrated that lay individuals, working with concernedmedical professionals, could manufacture, distribute, and test newtherapies with a minimum of side effects!
The amendments might have saved, at best, 7,000 lives. In contrast, many more died waiting the extra 10 years for life-saving drugs. According to my calculations, about 4.7 million people died over the last 40 years while the life-saving drug they needed was tied up in regulatory red tape!
Unfortunately, that’s just the beginning. The amendments have destroyed at least half of the industry’s innovative capacity, preventing some life-saving drugs from ever reaching the market.
For example, when I filed a patent for the treatment of fibrotic liver disease with prostaglandins, an FDA examiner called me personally. “You must encourage your company to develop this product,” he insisted. “We lose 100,000 people each year to fibrotic liver disease, and we have absolutely nothing to offer.”
The studies required by the Amendments, however, were especially long,difficult, and expensive. Studies with new, breakthrough drugs oftenare. If we guessed wrong the first time and had to repeat years and years of studies, our patent would run out and we’d never recoup our investment. In spite of the FDA’s support, we had to abandon this potentially life-saving drug.
The death toll from losing half of our innovations from 1962 to 2003 is somewhere between 4 and 16 million people depending upon theassumptions used. Adding the 4.7 million deaths due to an extra 10 years of development time suggests that as many as one out of three people who died of disease since 1962 may have done so needlessly.
The 1962 Kefauver-Harris Amendments may very well be the deadliest law that Congress ever passed.